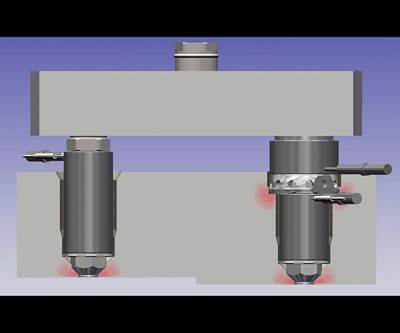
Like most of Integer’s tools, this two-cavity injection mold for a device called an introducer uses inserts to produce parts in seven different sizes and two different designs per size (14 total part numbers). The Cardiac Rhythm Management and Neuromodulation (CRM&N) team was struggling to validate the tool for certain part sizes due to filling issues. Eventually, a second tool (with minor parting line, runner and base-size changes to modify ejection) was produced and sent to an Integer facility in Mexico. While a team in Mexico worked on validating the new tool, Ed Flores in Minnesota focused on getting the original one to pass final testing. Photo Credit: Integer Holdings Corp.
With sales to over 50 countries, Integer Holdings Corp. is among the world’s largest contract medical device manufacturers, specializing in advanced medical technologies for the cardiac, neuromodulation, vascular and portable medical markets—including implantable devices. The company also supplies batteries for high-end niche applications in the medical, energy, military and environmental segments.
At its Cardiac Rhythm Management and Neuromodulation (CRM&N) facility in Plymouth, Minnesota, a team of 350 design and produce everything from custom medical components to fully packaged implantable devices, including supporting parts and tools. In addition to making most of its injection molds (≈100/year), the company extrudes tubular components and produces a broad range of thermoplastic parts via two-shot injection molding, insert molding and micro molding. Typical production runs range from 1,000 to 50,000 parts/year. Most molds feature only one or two cavities but use inserts to enable a single tool to produce multiple part sizes and designs.

Integer Holdings Corp.’s commitment to world-class manufacturing centers on lean facilities equipped with state-of-the-art technology. Eighteen manufacturing sites are ISO 13485 certified and 11 are FDA registered with approval to manufacture Class I to Class III medical devices. The company’s New Ross, Ireland, plant has twice received the prestigious Shingo Bronze Medallion for operational excellence. Shown here is the micro-molding area at the CRM&N facility in Plymouth, Minnesota. Photo Credit: Integer Holdings Corp.
Troubleshooting Challenges
Ed Flores, Integer CRM&N senior process engineer, uses scientific molding practices to develop implantable medical devices, including writing protocols, validating products and equipment, statistically analyzing results and running continuous improvement projects on existing tools. Before joining Integer eight years ago, he spent almost 18 years developing molding processes at an automotive contract parts supplier.
Flores’ first project at Integer involved troubleshooting a two-cavity injection mold for a device called an introducer, which features an overmolded polymer tube and two thermoplastic wings designed to break away/peel apart after device insertion. Like most Integer molds, this tool uses inserts to produce introducers in seven different sizes and two different designs per size, totaling 14 different part numbers. The critical medical parts must pass rigorous testing and process capability and performance (Ppk) requirements to ensure they are molded as designed and to spec. The CRM&N team was struggling to validate the tool for certain part sizes due to filling issues, which caused high scrap and higher frustration. Eventually, a second tool was produced by Flores’ predecessor and shipped to Mexico, where a team there worked to validate it while Flores focused on getting the original one to pass final testing.
“Technically, this mold had a geometrically balanced runner system, meaning each of the two gates feeding each part, and each runner feeding those gates had the same dimensions as corresponding runners and gates on the other cavity,” explains Flores, who says that despite moldfilling predictions to the contrary, parts didn’t fill evenly in certain part sizes. “The two outside gates were filling parts faster. We measured a 10% variance from one side of the part to the other and a 5% variance between cavities in these sizes. Flow imbalances meant we were fighting multiple issues, forcing us into a very tight processing window. If one part had good break force, then the part in the other cavity had poor handle integrity or vice versa.”
Using his training, Flores identified three design flaws likely contributing to introducer part failures: unbalanced fill, gate locations and process/material variances.
Flores had previously attended a molding workshop taught by John Beaumont, retired chair/professor of engineering at Penn State Erie, The Behrend College’s Plastic Engineering Technology program and founder of injection molding consultancy Beaumont Technologies Inc. (both located in Erie, Pennsylvania). At that workshop, Flores learned about a rheological control method invented by Beaumont called MeltFlipper, which corrects viscosity variations in multi-cavity tools that otherwise can lead to long cycle times, high scrap and shrink/warpage issues. It’s a technique Flores relied on at his previous job and introduced to Integer.

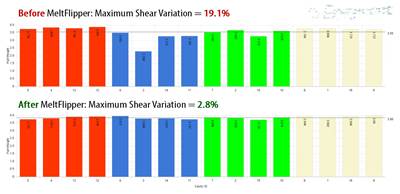
Using his AIM PTE training, Ed Flores identified three design flaws likely contributing to part failures in the introducer tool: unbalanced fill, gate locations and process/material variances. Starting with the part giving the greatest trouble among the 14, the team changed a runner plate on the three-plate tool to introduce rheological controls to balance fill by controlling polymer shear thinning in runners and gates. Remarkably, that one change resolved most of the tool’s issues and enabled the part with the most significant problems to finally be certified. The team filled its first production order for that part in late December 2021 without issue. Photo Credit: Integer Holdings Corp.
Flores later learned about the Plastics Technology & Engineering (PTE) workshop offered by Beaumont Technologies’ sister company, the American Injection Molding (AIM) Institute, which John Beaumont co-founded. “Since I’d been so impressed with John’s knowledge and background in plastic design and molding at the earlier workshop, and I’d heard such good things about the quality of AIM instructors, I made it a personal goal to attend that program and had been asking my manager,” Flores recalls. “Even though we produce and use a lot of injection molds, our company is not just a molder or moldmaker but also a medical device company. My previous manager simply didn’t know the reputations of AIM founders and teachers like John Beaumont, Mike Sepe and John Bozzelli.” Integer’s commitment to make the Plymouth facility its Molding Center of Excellence made it easy for Flores’ new manager to approve his request. He began the PTE course in 2019, finishing just before the pandemic lockdown.
Adult Learning Programs
John Beaumont, retired chair/professor of engineering at Penn State Erie, The Behrend College’s Plastic Engineering Technology program, founder of injection molding consultancy Beaumont Technologies Inc. and American Injection Molding (AIM) Institute co-founder, instructs students about various aspects of mold cooling design. Photo Credit: American Injection Molding Institute
The AIM Institute was founded in 2014, soon after John Beaumont retired from Penn State, and offered its first class—the PTE program—in 2015. At the time, Beaumont recognized how few options there were for industry professionals to learn more about plastics without returning to universities to pursue formal degree programs. AIM’s courses were designed to address that need by providing practical, proven adult learning programs for the injection molding industry. In the case of PTE, that involves attending four week-long physical courses at AIM’s facility. After returning home, students have several hours of virtual lessons each week during the two months between physical courses in Erie. The material taught by institute instructors is said to have been thoroughly researched by them or other institute members and each instructor performs ongoing research to keep course content updated.
“AIM courses are designed to highlight the difference between education and training,” explains David Hoffman, AIM director and instructor. “For example, we recognize that quality education takes time. We use multiple instructors per course and dive deep into the subject matter, pushing students to develop critical thinking skills. Our non-commercial content combines lectures, labs, demos and class exercises supported by homework assignments, weekly online reviews and additional learning opportunities. We assess progress using written exams and skills assessments, where applicable. Regardless of actual job functions, students completing PTE will be extremely knowledgeable in each of the four pillars of plastic materials, mold design, injection molding and part design.”
“We tell our students ‘Trust the process.’ That means trusting both the molding process and the education process.”
In addition to the PTE program, all three courses under AIM’s Molders’ Series are now ANAB accredited and ANSI/ASTM E2659 compliant, following ISO/ICE 17011. These standards require content to be reviewed by oversight committees, including an advisory board of 25 companies in AIM’s case. They also use formal ADDIE instruction design models, define learning outcomes and use psychometric assessment tools to measure learning. “We pursued third-party accreditation because we believe it’s an essential part of high-value education and training, and 80% of those we surveyed agreed,” adds Hoffman. The institute offers a variety of education options ranging from four-hour introductory online “boot camps” to the nearly year-long PTE program.

David Hoffman, AIM Institute director and instructor, discusses gate locations and various hot runner components with students.
Photo Credit: American Injection Molding Institute
“We tell our students ‘Trust the process,’” adds Hoffman. “That means trusting both the molding process and the education process. The courses and programs may seem daunting at first, but if students follow through and trust the process we’ve put together, they’ll learn more than they ever thought possible.”
Knowledge Boost
“Before taking the PTE program, I considered myself a subject matter expert with 27 years of experience in injection molding and a strong background in tool and die,” recalls Flores. “Honestly, I expected to breeze through at least a couple PTE courses, but boy was I mistaken. Until I took the course, I didn’t appreciate the depth and breadth of knowledge we’d be taught. I not only gained a much better understanding of injection molding but was excited to start applying what I’d learned.” As he completed each course, Flores wrote management summaries describing the purpose, scope, outline and takeaways, often listing planned changes and anticipated cost savings.
Using his training, Flores identified three design flaws likely contributing to introducer part failures: unbalanced fill, gate locations and process/material variances. Starting with the part giving the greatest trouble among the 14, his team changed a runner plate on the three-plate tool to introduce rheological controls to balance fill by controlling shear thinning of polymer flowing between runners and gates. Remarkably, that one change resolved most of the tool’s issues and enabled the part with the greatest problems to finally be certified. “By controlling rheology, we achieved balanced fill and pack in each cavity, which broadened our processing window to ensure both breakaway force and handle integrity were within tolerance,” Flores recalls. “This change didn’t alter the part’s design, specification or process. It only balanced plastic flow by controlling shear.” The second introducer tool has since returned from Mexico for retrofit with rheological controls.
Thanks to the knowledge gained from the PTE program, Flores has been recognized and compensated for his contributions. He went from being an individual engineer in a single facility to supporting two facilities and four departments. Meanwhile, a technician in Flores’ facility has completed the Molding 2 course at AIM and a manufacturing engineer is taking the PTE program.
“Honestly, I expected to breeze through at least a couple PTE courses, but boy was I mistaken. Until I took the course, I didn’t appreciate the depth and breadth of knowledge we’d be taught.”
Asked about the most valuable aspect of the program, Flores says access to the deep knowledge of instructors—both during and after the course. For example, students brought examples of problem projects to class for AIM instructors to troubleshoot during the program. “I was working on another project with uneven filling and thought I needed to use rheological controls to fix the problem,” he recalls. “I showed it to Dave [Hoffman], who studied the part and short shot data for a few minutes and determined the problem was that the core pins were not on center. Sure enough, we measured the tool and they were off. Being able to access that kind of knowledge is the institute’s strongest selling point.”
“I survey our students every year and Ed’s story is not uncommon,” adds Hoffman. “Students often tell us about promotions, pay raises and other benefits coming directly from cost-saving ideas they implemented based on what they learned here at AIM. As instructors, that’s very rewarding and tells us we’re doing something right.”
Related Content
Hands-on Workshop Teaches Mold Maintenance Process
Intensive workshop teaches the process of mold maintenance to help put an end to the firefighting culture of many toolrooms.
Read MoreMaking Mentoring Work | MMT Chat Part 2
Three of the TK Mold and Engineering team in Romeo, Michigan join me for Part 2 of this MMT Chat on mentorship by sharing how the AMBA’s Meet a Mentor Program works, lessons learned (and applied) and the way your shop can join this effort.
Read MoreHow to Foster Innovation Through a Culture of Education, Mentoring
Dynamic Tool Corp. shares its strategy for building a team with the right attitude and aptitude to deliver innovation that meets customer expectations.
Read MoreTackling a Mold Designer Shortage
Survey findings reveal a shortage of skilled mold designers and engineers in the moldmaking community, calling for intervention through educational programs and exploration of training alternatives while seeking input from those who have addressed the issue successfully.
Read MoreRead Next
Choosing Between Cold and Hot Runner Systems
Get all the facts before selecting a runner system solution.
Read MoreDigging into Data to Design Out Fill Imbalance Problems
Injection molder uses a custom runner design to reduce shear-induced imbalances and control shrink and warp, dimensional instability, and shot-to-shot inconsistency.
Read MoreSix Considerations for Evaluating a Hot Runner System
Details matter when it comes to selecting and integrating a hot runner system. This guide makes that process easier by covering those details thoroughly.
Read More

.jpg;width=70;height=70;mode=crop)