Making the Most of High-Performance Mold Materials
Understanding high conductivity alloys and optimizing their use can help you build better molds.
Injection molders and blow molders can benefit from high conductivity alloys by achieving faster cycle times and better part quality. There are certain properties of the mold material and polymer that enable these efficiencies to be realized. Once these characteristics are understood, mold builders can optimize their use of high-performance materials to provide a durable, fast-cycling mold for their customers.
Cooling Time
Mold Alloy Thermal Properties
Some characteristics of mold materials enable us to better understand the thermal process that occurs while molding. Three important properties are:
1. Thermal Conductivity
Higher thermal conductivity equates to the transfer of more thermal energy per unit of time under steady state conditions.
2. Thermal Diffusivity
Higher thermal diffusivity means that thermal equilibrium will be reached faster when the temperature changes. A good thermal diffuser will react more quickly to environmental temperature changes.
3. Thermal Effusivity (conductivity divi-ded by the square root of the diffusivity)
Higher thermal effusivity is a measure of the material’s efficiency at instantly removing heat from an object at a higher temperature with which it suddenly makes contact (see Chart 1).
| Chart 1 | |||
| Mold Material | Thermal Conductivity, W/(cm-oK) | Thermal Diffusivity, cm2/sec |
Thermal Effusivity |
| Steel - P20 |
0.29 | 0.081 | 1.02 |
| Copper Beryllium | 1.25 | 0.415 | 1.94 |
The following explains what all of this means when molding plastics.
1. Heat mold up to operating temperature (via water channels).
- The higher diffusivity allows the copper mold alloy to reach equilibrium faster, so the molding operation can begin sooner.
2. Inject hot plastic melt into the mold and cool.
- Higher effusivity means the mold will begin to instantly and efficiently remove heat from the plastic.
- Then the high diffusivity translates to reaching steady state, uniform temperature quickly.
- Finally, once at equilibrium the conductivity determines how fast the thermal energy will be removed from the plastic until the part reaches the desired ejection temperature.
3. Maintain setpoint temperature (equal to water temperature) during mold-open, ejection and mold-close portions of the cycle.
- Again, the high diffusivity enables the mold to maintain equilibrium at setpoint during mold open, ejection and mold close. Since the air is a poor thermal medium, the contact between the water and copper is the overriding factor.
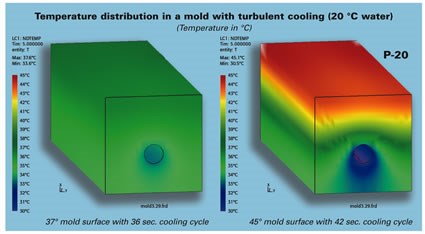
Figure 1 shows pictures from a thermal FEA illustrating the uniform temperature of a copper beryllium mold compared to that of a mold made of P-20 steel.
Polymer Types
The two main polymer families—semi-crystalline and amorphous—both benefit from higher conductivity mold materials.
Semi-crystalline polymers have a densely packed, uniform molecular structure and include materials such as polyamide (nylon), polyethylene, polypropylene and polyacetal. These polymers become amorphous when melted during processing and will become semi-crystalline again when cooled.
Amorphous polymers have a loose and random molecular structure, so that in some cases amorphous materials are transparent. Both types of polymers can benefit from improved heat transfer and reduced cooling time.
The following are some differences that need to be realized to provide a better understanding of the application.
- Crystalline materials have a sharp melting point, and thus a latent heat energy that must be added when melting, and removed when cooling. The plastic needs to be solidified and cooled below the heat deflection temperature before ejection from the mold. The heat deflection temperature (HDT) is available on most resin datasheets. Just getting below the melting point is not enough. The part has to be cooled to the point where it is stiff enough to eject. Glass and mineral fillers increase the crystallization rate and the HDT so the part can be ejected at a higher temperature without deformation.
- Amorphous polymers do not have a melting point, but as the heat input is increased above the glass transition temperature (Tg), the viscosity of the polymer decreases until it begins to flow. Heat is added until the plastic can flow adequately to fill the mold. Then the heat has to be removed until the polymer is below the Tg—in many cases before the part will be stiff enough to be ejected.
In general, crystalline polymers contain more heat energy due to the latent heat. For example polycarbonate—which is amorphous—has a heat capacity of 1.2 J/(g oK) while polypropylene—which is semi-crystalline—has a heat capacity of 1.9 J/(g oK) or 58 percent higher. Molders will experience cycle time reductions and improved uniformity of cooling for both families of plastics when using high conductivity mold alloys.
Some semi-crystalline materials—such as nylon—require relatively high mold temperatures to provide good surface finish and maximum crystallinity. High conductivity mold alloys can improve both characteristics, and reduce cycle time as an added bonus.
This effect is achieved by simply running the mold at the desired temperature—for example 180oF. The high conductivity alloy will be able to remove heat faster than steel, but at the recommended temperature, and the heat removal will be more uniform. The result is reduced cooling time and more uniform crystallinity in the molded part. When molding amorphous plastics, uniform cooling also is very important. For clear polymers—like polycarbonate—the part will have better clarity and toughness.
Water Cooling
With steel tools, molders often run chillers to reduce cycle times and to compensate for the reduced heat transfer of the steel. The cold tool will often result in condensation on the mold surface that can adversely affect part quality. With high-conductivity tool alloys, the cooling water can be set at a higher temperature to prevent condensation, and yet achieve much faster cycles than steel tools. Also, the surface temperature of the mold will be very close to the water temperature setpoint. The long-term heat transfer performance of copper alloys is very good, because copper resists corrosion and bio-fouling in the cooling channels.
Economics
Cycle time reduction always has been a key effort for molders. Increasingly, molders are attempting to improve cycles to offset higher resin, energy and transportation costs that they have not been able to pass through to their customers. Using copper mold alloys allows molders to improve their production rate, avoid capital investment and minimize quality issues.
Higher conductivity molds provide more uniform cooling than steel tools, resulting in better dimensional control, decreased warpage and part strength improvements. Payback analysis for molds using high-performance alloys yields very desirable numbers due to the reduced cooling times.
Applications
- Recently, in the case of a large polyethylene lid, the molder calculated the payback at 10 days using a copper beryllium insert in a steel tool. The cycle time was reduced from 75 seconds to 52 seconds, and allowed the molder to avoid purchasing an additional molding machine to keep up with demand. Capital avoidance is sometimes overlooked, but can be of tremendous benefit in the long term.
- Another example is a chair base made of glass-reinforced nylon. The manufacturer was able to obtain a 20 percent cycle time reduction using copper beryllium for a core insert in the chair base mold (see Figure 2). Prior to using copper beryllium, the manufacturer was using strictly steel in its molds. After switching to molds using copper beryllium inserts they have witnessed a decrease in cycle from 122 seconds to 98 seconds—allowing for faster production throughout. Also, the dimensional control of the hub diameter was improved.
By using copper beryllium, the manufacturer was able to increase annual production by 500,000 chair bases without purchasing additional injection molding machines. With all steel tools, four additional presses were required to meet the growing demand Again, major capital outlay was avoided for a minimal investment in high conductivity mold alloys.
Conclusion
The benefits of high conductivity alloys include (1) faster cycle times, (2) uniform mold temperature, (3) better part quality, (4) low-maintenance cooling channels, and (5) suitability for amorphous and crystalline polymer families. Mold builders that have expertise and capabilities with these mold alloys have a competitive edge in a global marketplace. Such moldmakers can offer their customers high-performance molds that will enable their customers to be more competitive and profitable. And we all know that profitable customers are the best kind.
Related Content
Technology Review and Sourcing Guide 2024: Mold Materials
Building a high-quality mold requires the proper selection of appropriate mold materials. Tool steel, aluminum, copper and alloys are a just a few examples you can fin in this exclusive, online-only content that includes a supplier list for mold materials.
Read MoreSolid Carbide Cutter Mills Aluminum
Roughing and finishing deep pockets and cavities are the primary feature strengths behind this Walter MC166 Advance cutting tool.
Read MoreCast Plate Aluminum Alloy is Suitable for Production Mold and Dies
Alimex Precision in Aluminum introduces its 7000 series ACP7 mold material, featuring high mechanical properties, material stability and good machinability.
Read MoreHow to Select the Right Tool Steel for Mold Cavities
With cavity steel or alloy selection, there are many variables that can dictate the best option for moldmaking.
Read MoreRead Next
A Copper-Nickel-Silicon-Chromium Alloy for Mold Tooling
A review of the physical and mechanical properties of one copper alloy system will help moldmakers better understand how this alloy will perform thermally and mechanically in applications.
Read MoreHow to Use Continuing Education to Remain Competitive in Moldmaking
Continued training helps moldmakers make tooling decisions and properly use the latest cutting tool to efficiently machine high-quality molds.
Read MoreHow to Use Strategic Planning Tools, Data to Manage the Human Side of Business
Q&A with Marion Wells, MMT EAB member and founder of Human Asset Management.
Read More