An Argument for Copper Conductivity
When looking for mold material with efficient heat conduction, moldmakers may do well to keep copper berylium in mind.
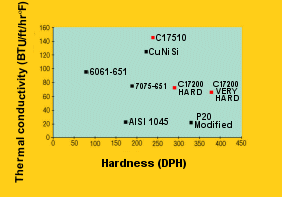
Mold material manufacturers advertise their material in terms of the properties that they believe will appeal to the mold designer. Attributes like hardness and conductivity are reported with some expectation of excitement on the part of the designer (see Figure 1). However, when it comes to conductivity, the numbers show that copper beryllium beats steel for faster cooling, hands down.
In the heat flow circuit, it is essential to keep in mind that the conductivity of the mold material at the plastic/metal interface is only about as good as the weakest link in the chain. This simply means that you may not be enjoying all of the heat conduction available in the mold material if there is a lower conductivity element "downstream."
A first step toward quantifying the value of conductivity in a mold system can be found in Go With the Flow: Mold Cooling Optimization From a Moldmaker's Perspective (MoldMaking Technology, October 2001). This article brought readers face to face with the realities and importance of dealing with cooling capacity in the early stages of mold design with emphasis on cooling water flow.
In this article there is an excellent description of the requirements for cooling water flow and plumbing philosophy. It says that for a given part, the ability of the mold material to carry the necessary heat away from the plastic and into the cooling water depends on three primary factors:
- The thermal conductivity of the material.
- The difference in temperature between the plastic/mold interface and the mold/water interface.
- The distance between those interfaces.
The governing equation is: q/A = k(∆t/∆s) where:
- q is heat flow in BTU/hr.
- A is area through which heat flows in square feet.
- k is thermal conductivity of the mold material in BTU/ft/hr/'F.
- ∆t is the temperature difference through the thickness of the heat path (plastic to water).
- ∆s is the heat path distance.
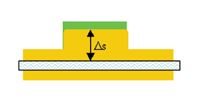
In Figure 2, heat must flow a short distance from the plastic part to the flowing water. If the plastic/mold interface is close to 450'F, and the mold/water interface is at 150'F, the ∆t is initially 300'F.
If ∆s is two inches (0.1667 ft.), and the material is P20 steel (k= 16.7BTU/ft/hr/'F), then the value for q/A is about 16.7*(300/0.1667) = 30,053 BTU/hr/ft}.
If the area through which the heat must pass to get to the water is 10 square inches (0.0694 ft}), that section of the mold will be able to extract 2,087 BTU/hr. If the plastic part in this area weighs , pound, and requires around 300 BTUs per pound to set, the cooling time will be 0.036 hours or just over two minutes.
However, consider that the same mold made from copper alloy C17200 (k=77.3 BTU/ft/hr/'F) would permit the part to set in 28 seconds!
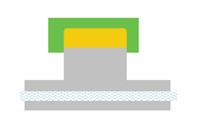
This is based on sufficient water cooling. But one additional complication arises if the heat must flow through more than one solid body prior to being carried away by the liquid. In Figure 3, a small section of the component is so far removed from any available cooling that the heat must pass through a second body prior to reaching the flowing coolant. In such a case, the heat flow behaves like a series electrical circuit, particularly in terms of resistance. The equation becomes a bit less attractive:
q/A = ∆t/(∆s1/k1+∆s2/k2).
The subscripted terms refer to the thermal conductivities and thickness of each of the two bodies in the conduction path. In this case, the distance is now 3.5 inches (0.2917 feet). This alone will slow the cooling time in a beryllium copper component from 28 seconds to 49 seconds.
If, however, the heat path consists of 1.5 inches of beryllium copper, followed by two inches of steel, the cooling capability slows to over 2.5 minutes.
Real World Applications
For as long as there have been schools, textbook physics problems have been full of massless-frictionless pulleys, zero-resistance wires and infinite heat sinks. These books represent (or rather, try to hide) the point that the real world rarely behaves in accordance with simple equations because the assumptions are often not appropriate.
The predicted times calculated above are likely to be somewhat shorter than actually observed. Some things to consider are:
- As with electrical circuits, there is additional resistance at the interface between the two bodies mentioned above. The importance of this is easily underestimated. Even with very smooth surfaces, there is a surprisingly small amount of area where the bodies are actually in contact. To minimize this impact if the heat is forced to travel through more than one body to get to the water, make the interface very tight.
- The water/mold interface is rarely as large as the plastic/mold interface. Use baffles, turbulence, water temperature and water volume to the greatest advantage.
Know the Facts
Based on the calculations above, it seems puzzling that any mold would be designed without serious consideration for beryllium copper as the primary material for difficult cooling problems, if not for the whole mold. Some mold builders shy away from beryllium copper due to some of the sensationalized articles about beryllium and illness related to overexposure. However, guidelines for safe machining, polishing and electric discharge machining of beryllium copper are readily available from the alloy manufacturer.
The following is not intended as a substitute for those guidelines, but may help dispel some of the typical misconceptions:
- Beryllium copper alloys contain very low levels of beryllium - generally between 0.5 and 1.85 percent. These materials pose no threat in solid form.
- Beryllium is a naturally occurring metal element found in various gemstones, minerals and soils throughout the world.
- The greatest release of beryllium into the air is from combustion of fossil fuels.
- Because of its strength, conductivity and other properties, beryllium copper surrounds us as components in computers, cars, phones, toys and countless other devices.
- Beryllium is not radioactive.
- Beryllium copper in solid form does not emit beryllium "vapors."
- Machining coolant does not become a hazardous waste after machining beryllium copper.
- Beryllium copper has not been banned in Europe or any other country.
- "Beryllium free" copper alloys do not possess equal performance capability, and contain other elements with well-documented detrimental health effects.
A decision based on fact and calculation will always have a competitive edge over one based on fear and custom.
Read Next
A Copper-Nickel-Silicon-Chromium Alloy for Mold Tooling
A review of the physical and mechanical properties of one copper alloy system will help moldmakers better understand how this alloy will perform thermally and mechanically in applications.
Read MoreHow to Use Continuing Education to Remain Competitive in Moldmaking
Continued training helps moldmakers make tooling decisions and properly use the latest cutting tool to efficiently machine high-quality molds.
Read MoreReasons to Use Fiber Lasers for Mold Cleaning
Fiber lasers offer a simplicity, speed, control and portability, minimizing mold cleaning risks.
Read More